
6. Label the given diagram of Bunsen burner with the phrases given below. Brainly.in
CH4 + 2O2 Equation 1 CH4 + O2 CO2 + 2H2O + heat C + 2H2O + heat Equation 2a CH4 + 3⁄2O2 → CO + 2H2O + heat Equation 2b The modern Bunsen burner has changed very little from Robert Bunsen's original design.
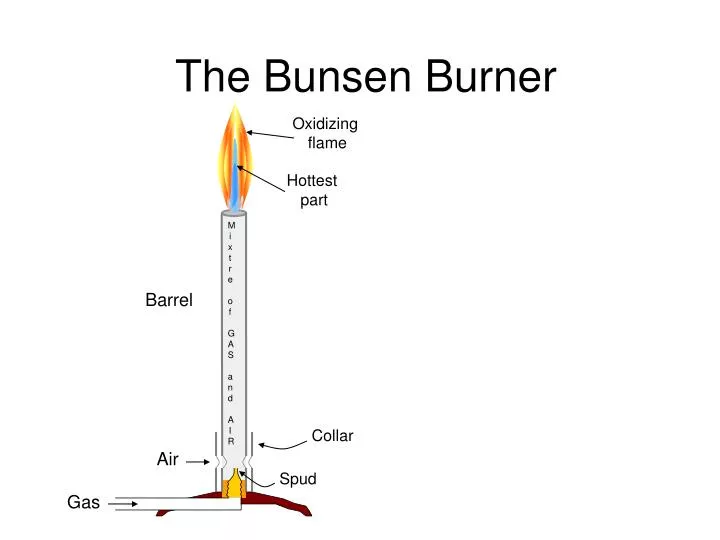
PPT The Bunsen Burner PowerPoint Presentation, free download ID5832205
The structure of Bunsen cone flame tops is studied using the thermal diffusion flame model. Assuming a strong temperature dependence of the reaction rate, the shape of the flame front as well as the temperature distribution at the front are found. It is shown that for the case in which the Lewis number of the limiting component is greater than.
Bunsen Burner Flame Diagram Wiring Service
The most common flame atomization device, which is illustrated in Figure 6.2A. 1 6.2 A. 1, is known as a laminar flow or pre-mix burner. Note the unusual design of the burner head, which instead of having the shape of a common Bunsen burner, has a long, thin flame that is 10 cm long. Radiation from the course passes through the 10 cm distance.
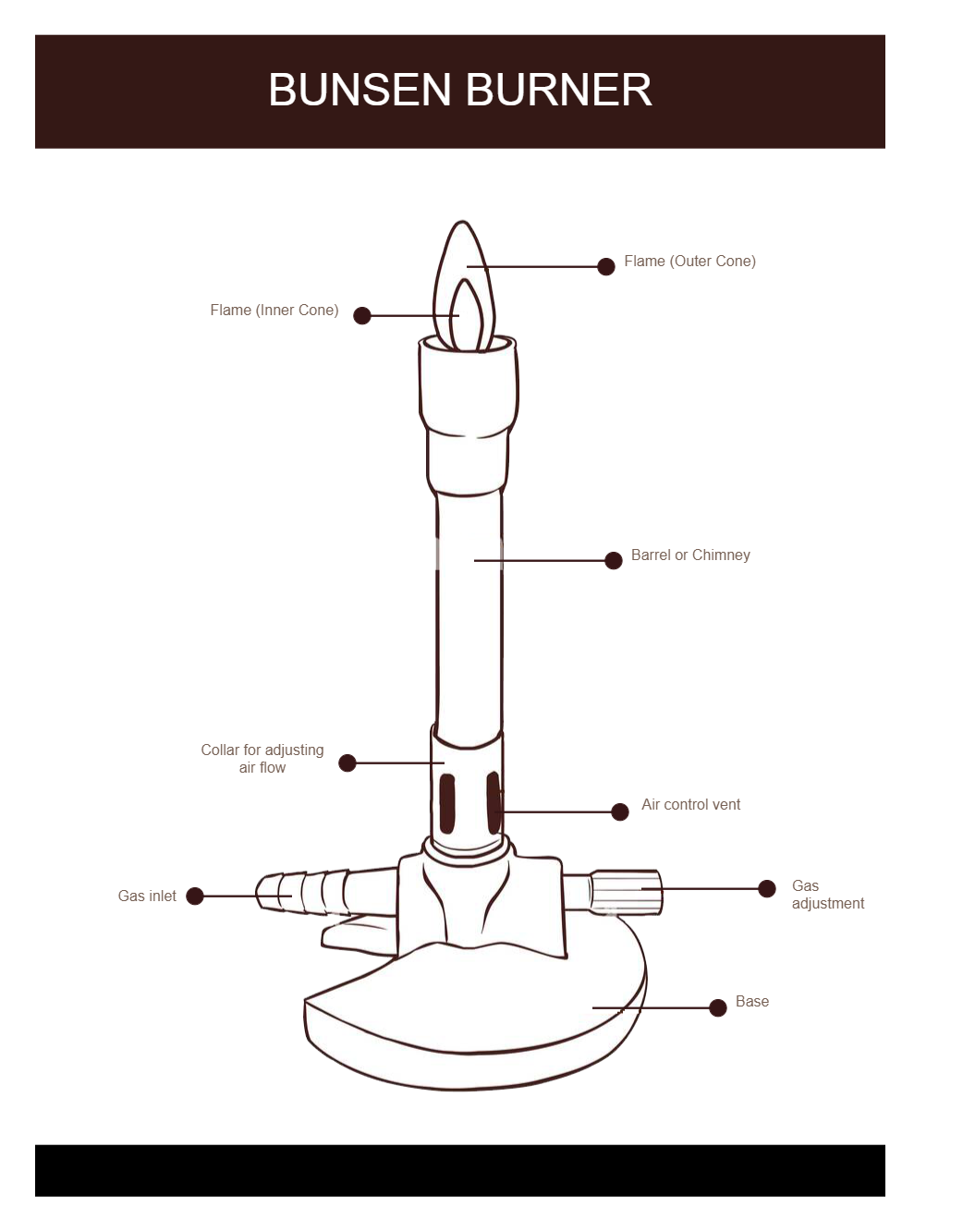
Bunsen Burner Diagram AfsheinLakota
To light a burner; Bunsen burners are generally used to rapidly heat high-boiling liquids with low flammability (such as water). Safety note: It is important to know that they can reach temperatures of approximately \(1500^\text{o} \text{C}\),\(^5\) and can easily ignite most organic compounds. If an apparatus is improperly set up, or if there is a small gap that allows organic vapors to.

Science bunsen burner diagram illustration Stock Vector Image & Art Alamy
A Bunsen burner, named after Robert Bunsen, is a kind of ambient air gas burner used as laboratory equipment; it produces a single open gas flame, and is used for heating, sterilization, and combustion. [1] [2] [3] [4] [5] The gas can be natural gas (which is mainly methane) or a liquefied petroleum gas, such as propane, butane, or a mixture.
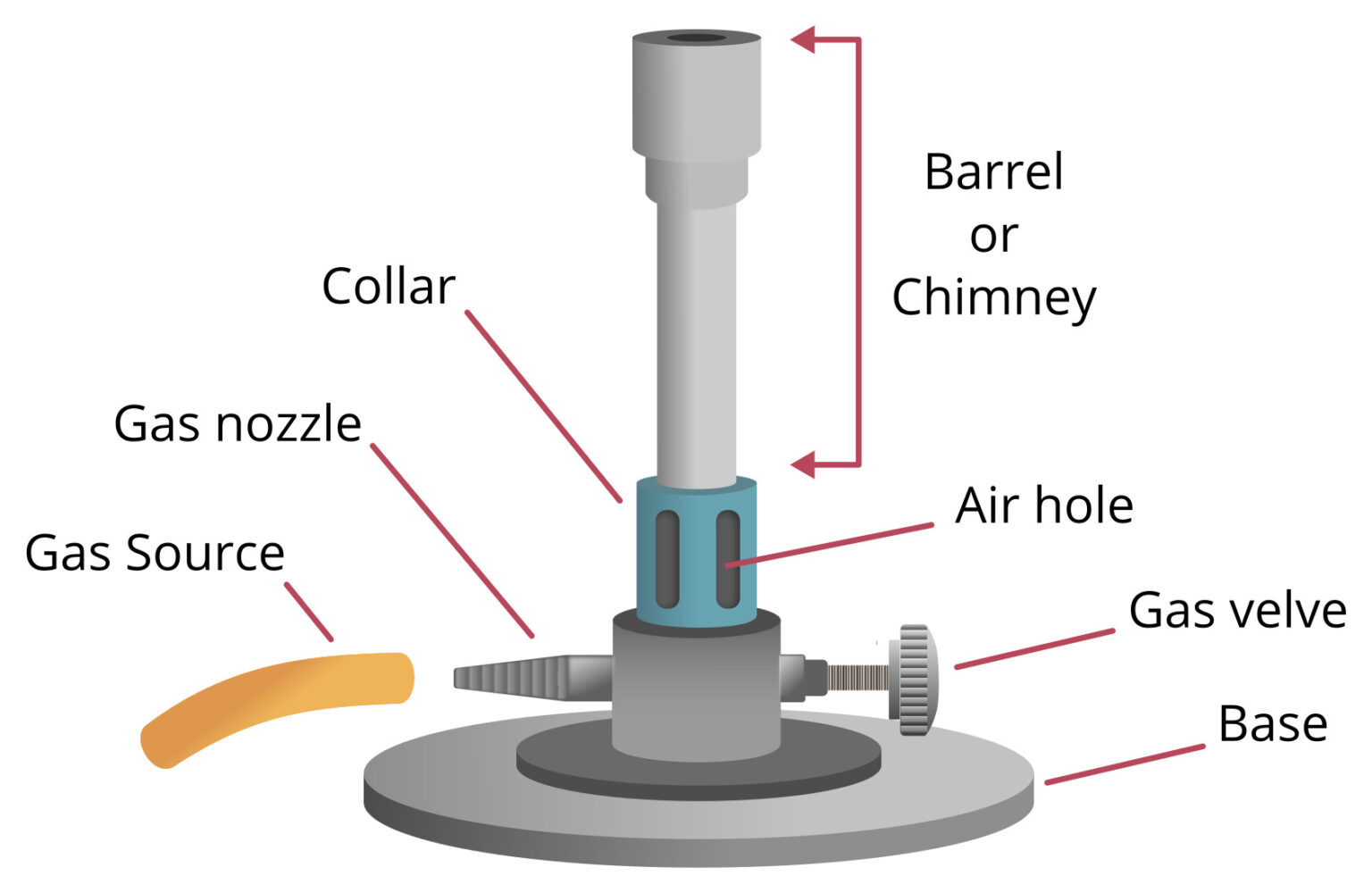
Bunsen Burner Definition, Principle, Parts, Functions
Bunsen Burner: Definition, Parts, Types and Uses. February 22, 2023by Adeel Abbas. Written By Adeel Abbas. Bunsen Burner is a laboratory device that is widely used in scientific research, teaching, and experimentation. It is a simple gas burner that produces a hot, blue flame that is perfect for heating, sterilizing, and combustion purposes.

Types of Bunsen burner flames YouTube
The Bunsen burner is the object most frequently associated with a chemistry laboratory. In this lab, it will serve as the primary heat source. The burner operates on natural gas,. Lighting the burner in this manner will result in a flame that is 1-2 inches tall. Normally a flame size of 3-5 inches is needed. Gas flow into the burner tube.
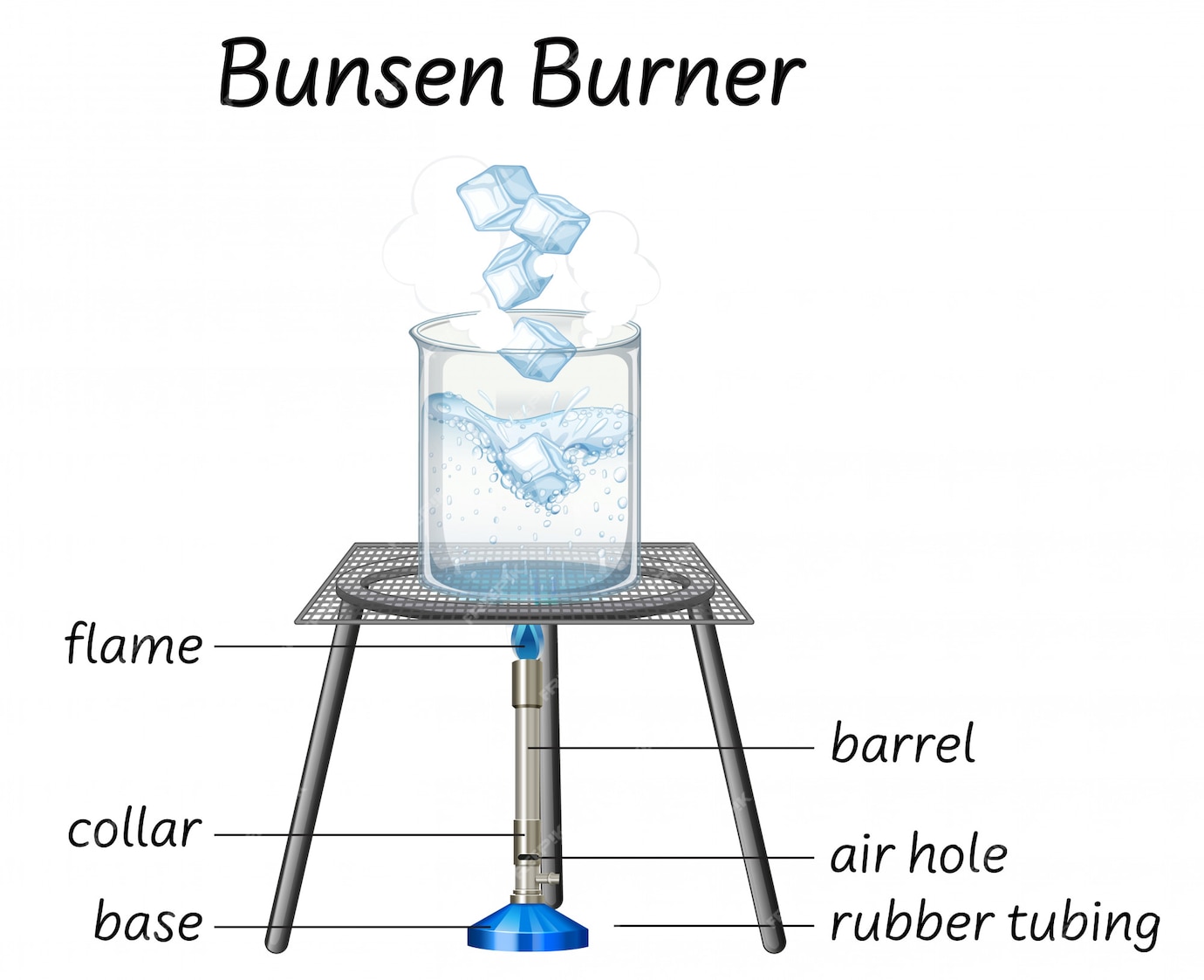
Free Vector Science bunsen burner diagram
A Bunsen burner flame sits at the mouth of the tube at the top of the equipment, and can be effective for creating a convection current. Standard natural rubber tubing has been used for many years, but you can also use tubes made from neoprene or a tube made from a polymer blend like Enduraflex.
Parts Of A Bunsen Burner Diagram Images and Photos finder
Bunsen burner is a gas burner that produces smokeless, nonluminous flame used for heating, sterilizing, and combustion purposes in laboratory experiments. It was named after Robert Bunsen, a German scientist who designed it in 1857. A.D. Bunsen burner ignites by the fusion of fuel and air (oxygen).

33 Label The Parts Of The Bunsen Burner Labels 2021
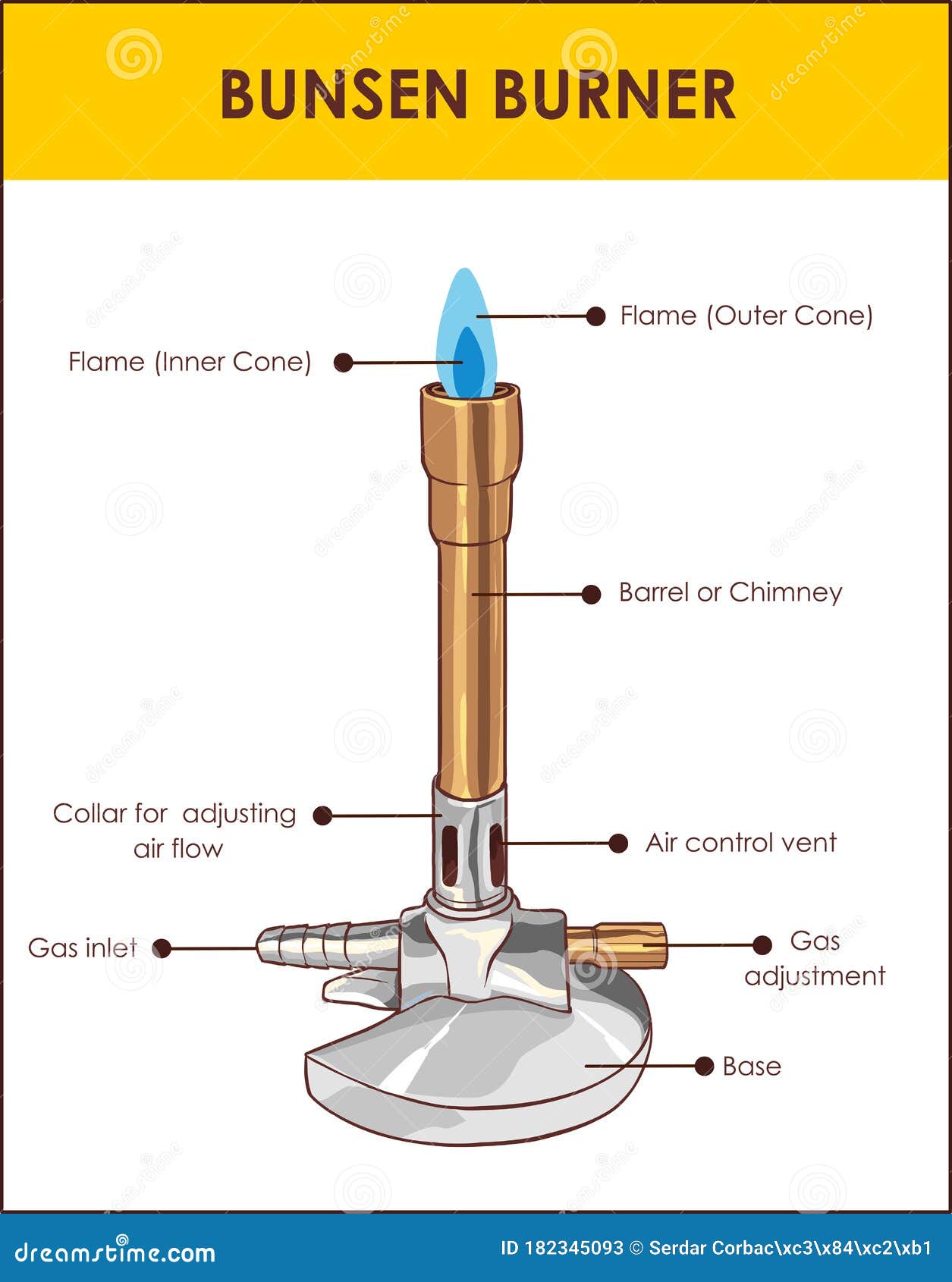
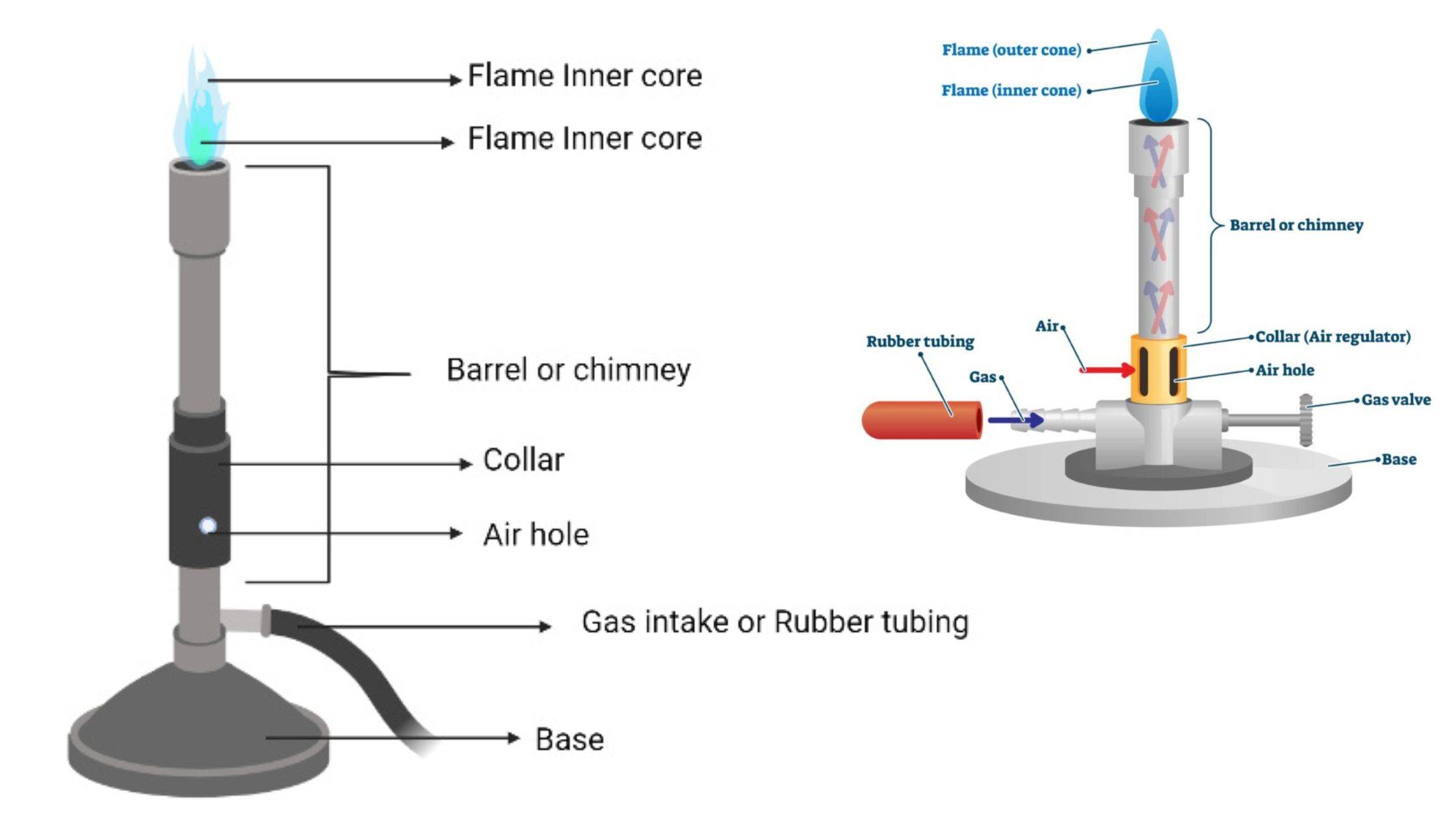
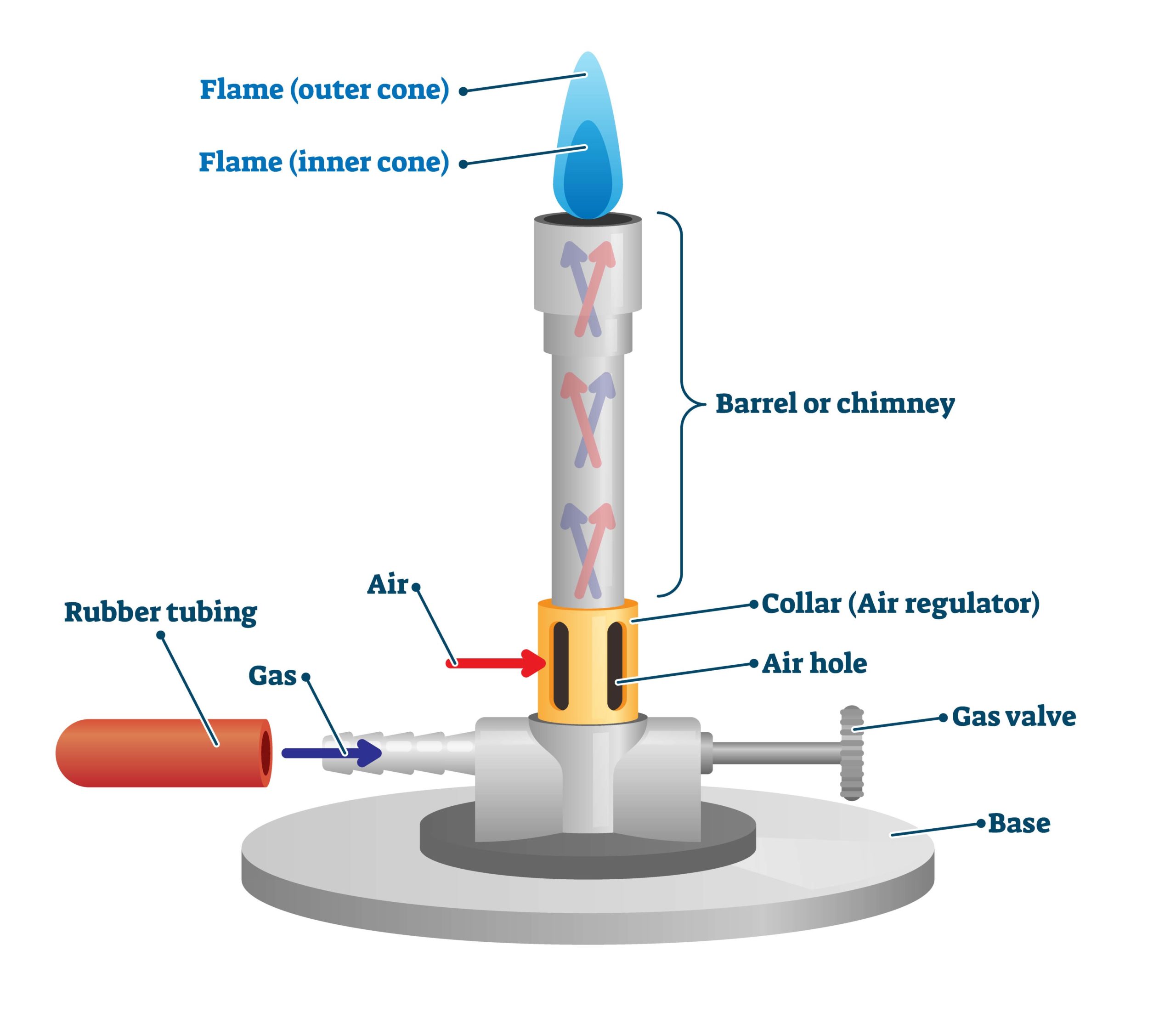
However, one should be familiar with the different parts of a burner to handle it safely and understand how it works. An efficient Bunsen burner is purely metallic (except the gas tubing) and has five main parts: 1. Barrel or stack: It is approximately 5 inches long to raise the flame to a suitable height for heating.
PPT The Bunsen burner PowerPoint Presentation, free download ID6216773
A Bunsen burner is a laboratory device that plays a crucial role in scientific experiments and research. It was invented by German scientist Robert Bunsen in collaboration with his lab assistant Peter Desaga in 1857. The burner was named after Bunsen, recognizing his contribution to its design and development.
Bunsen Burner Working, Parts, Types and Uses
A typical diagram is shown below: In this article you'll learn: What are the parts of a Bunsen burner? What is the function of a Bunsen burner? How do you use a Bunsen burner? What is the hottest part of a flame on a Bunsen burner? Let's Get Started…! Parts of Bunsen Burner and Their Functions

How to Draw Bunsen Burner labelled Diagram Bunsen Burner lab Equipment ! YouTube
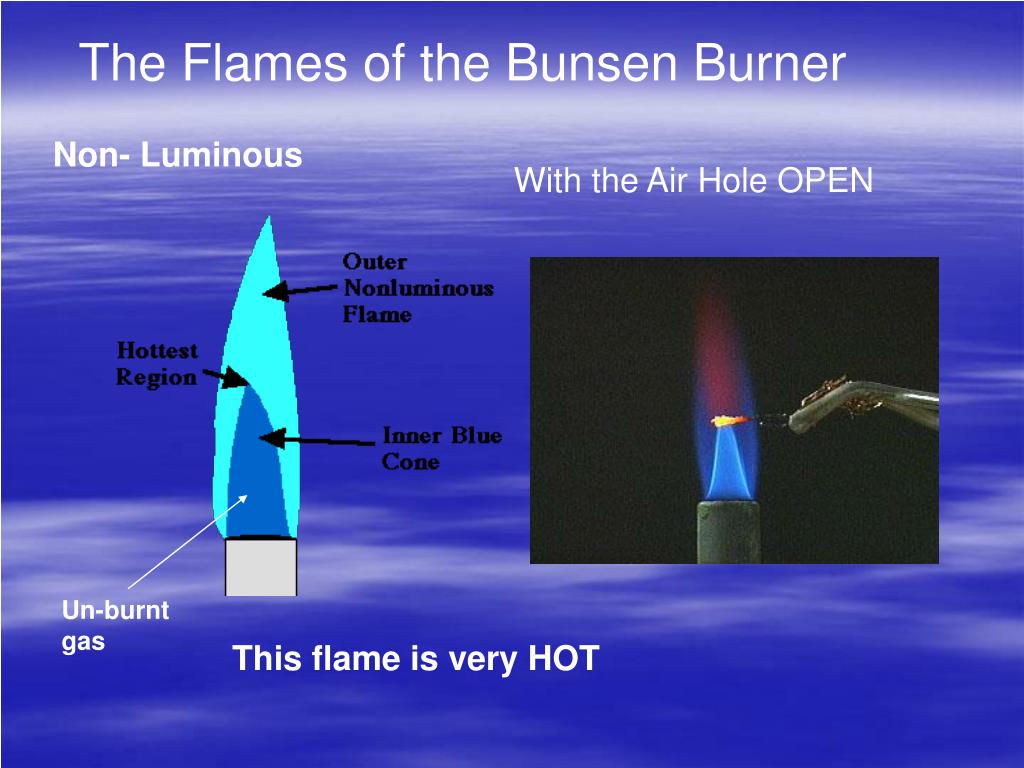
Diagram below the three distinct flames you can create with the Bunsen Burner: In your diagram label the different temperature regions, if necessary. Flame #1: Temp: ________________ Flame #1 Diagram Color: ________________ Observations: Flame #3 Diagram Flame #2: Temp: ________________ Color: ________________ Observations: Flame #2 Diagram
Bunsen Burner Definition, Principle, Parts, Functions
Watch and learn how to light a bunsen burner and observe the different flames produced by a bunsen burner. Luminous flame and non-luminous flame. Learn more. [email protected] +254721524786 . Home; Chemistry. Draw and label the diagram of a luminous flame as seen in the video. Why should the collar be closed for this flame to occur?
Bunsen Burner Function ShawnatRich
1. Get a Bunsen burner and a piece of rubber tubing for Bunsen burners. Check for any cracks or damage on the rubber tubing. Get a new piece of tubing if yours is damaged. Attach one end of the tubing to the gas source on the laboratory bench and the other end to the gas inlet on the Bunsen burner. 2.
Science project no.4 Science, Bunsen Burner ShowMe
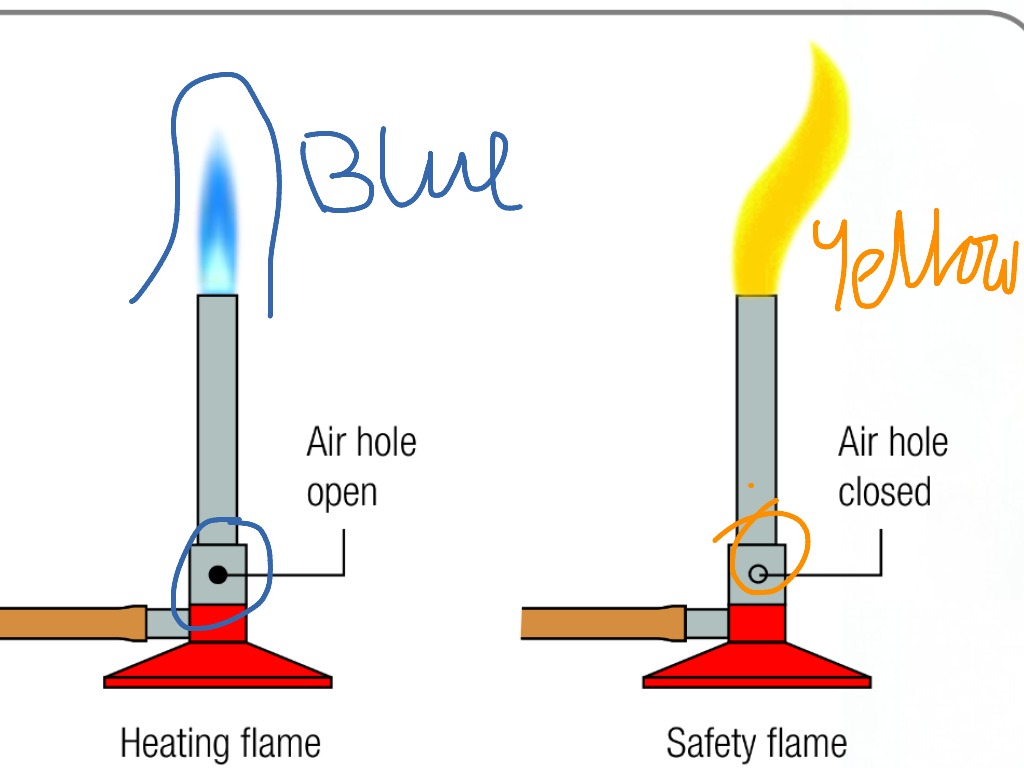
Combustion, Reactive Hazard, and Bioprocess Safety. Shijie Liu, in Bioprocess Engineering (Second Edition), 2017. 18.8.3 Diffusion Flames. When the air inlet is shut off in a Bunsen burner, the flame can still be maintained, but it switches from the blue color of a normal CH 4 flame to the yellow of a diffusion flame. This is the situation of a gas stove. Pure CH 4 flows up the tube, and the O.